Non-communicable diseases (NCDs), such as neurodegeneration and cancer represent the leading cause of death worldwide, killing an estimated 41 million people each year—equivalent to 71% of all deaths globally. Although our understanding of NCDs has advanced substantially in recent years, treatment often remains challenging due to the complexity and limited availability of sensitive screening tools for NCDs. Therapeutic interventions furthermore often require rapid and robust reporting of key biomarkers to adjust treatment according to the molecular defect to be effective. In this regard, autophagy, a key intracellular protein degradation process, is an attractive therapeutic target. However, measuring the process of autophagy is challenging and often requires labour-intensive techniques. In this article, we explain the phenomenon of autophagy, discuss the challenges to assess it and touch briefly on a proposed solution to the challenges that is currently being developed by a company called Phagoflux™. The Phagoflux team comprises Prof Ben Loos (CEO of Phagoflux, Department of Physiological Sciences), Prof Willem Perold (Department of Electrical and Electronic Engineering), Prof Jan-Hendrik Hofmeyr (Department of Biochemistry), Dr Andre du Toit (Department of Physiological Sciences) and Prof Pieter Fourie (Innovation for Life). The former engineering student Christiaan Viviers conducted his MSc work focussing on the device described below.
1. What is autophagy?
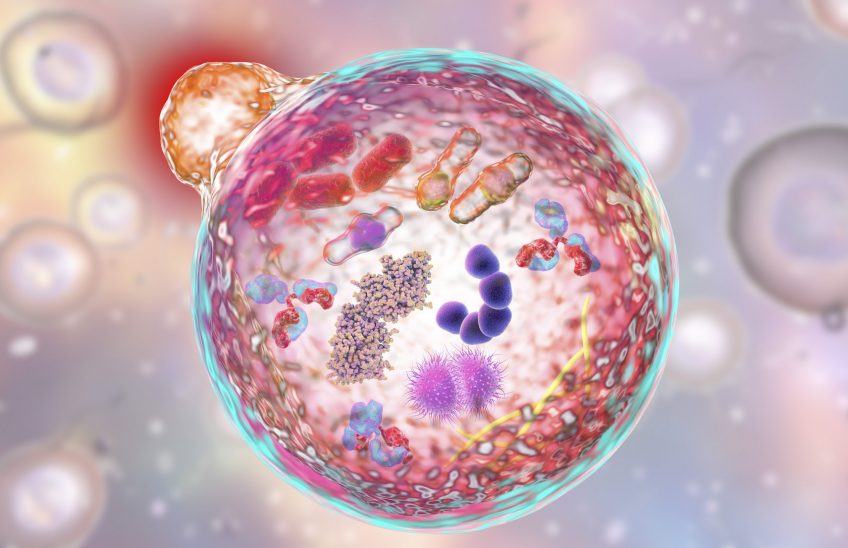
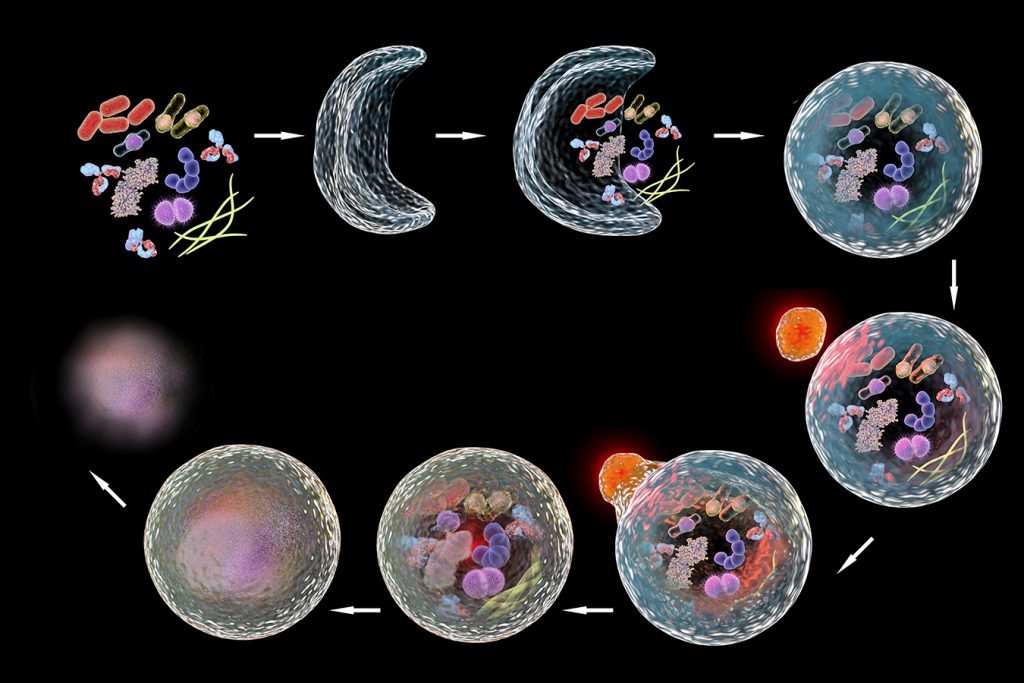
Autophagy means “self-eating.” It is a metabolic process responsible for the degradation of cellular long-lived proteins and organelles and plays a vital role in cellular function by maintaining proteostasis and removing harmful proteins. Put simply, autophagy is the body’s way of cleaning out damaged parts in cells, thereby allowing the regeneration of newer, healthier parts and components in cells. It is an evolutionary self-preservation mechanism through which the body can remove dysfunctional components and recycle parts of these cells in the process of cellular repair and cleaning. However, an abnormal deviation in this process has been linked to several diseases, most notably neurodegenerative diseases such as Alzheimer’s disease or Parkinson’s disease, where specific proteins accumulate and lead to neuronal toxicity and ultimately cell death.
2. Challenges to assessing autophagy
As mentioned above, autophagy is an attractive therapeutic target with several exciting techniques used to assess it, such as western blot analysis, electron- and fluorescence microscopy. However, these techniques are often labour intense, qualitative, and require significant expertise for execution. This makes them largely unsuitable to be employed in a clinical setting where the screening of autophagy modulating drugs would be most required. Moreover, neurodegenerative diseases occur more frequently with a global ageing population which further enhances the need for technologies to accurately and rapidly assess autophagy-related proteins.
3. The proposed solution
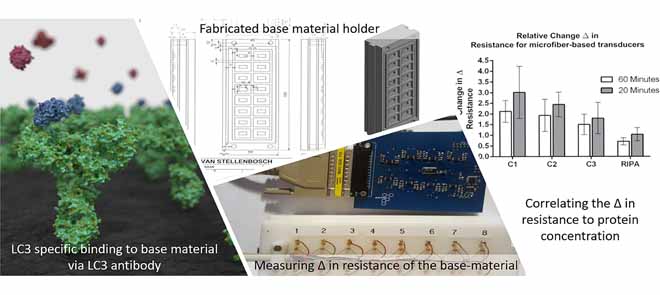
“[A] resistive-based biosensor … that utilizes conductive microfibers to detect microbial pathogens” was initially designed to address these challenges. This design was expanded for the rapid detection of the abundance of proteins. In particular, a biosensor was developed that is capable of measuring microtubule-associated protein 1A/1B light chain 3 (LC3), a structural protein incorporated into the membrane of autophagosomes, and generally used as a marker protein of the autophagy process. Microfiber-, paper- and CNF-based transducers were developed and incorporated into a resistive-based sensor that measures protein abundance associated with autophagy, bound to immobilised LC3 antibodies. The microfiber- and paper-based transducers were functionalised using PPy composite before antibody immobilisation, enabling electron flow through the base material and allowing for the detection of LC3 protein abundance by detecting a change in resistance. In contrast, the CNF-based transducer did not require additional modification and proved to be more sensitive than the microfiber- and paper-based transducers. While the detection of specific proteins by antibodies and the quantification of biomolecules through resistive sensing is not necessarily novel, the unique integration of resistive sensing with hardware and software design enabling the detection and monitoring of autophagy is uniquely powerful.
4. Conclusion
We are just beginning to understand how autophagy works in the body and what we have learnt so far is compelling. Autophagy will continue to gain attention as researchers, such as the team at Phagoflux, conduct more studies on its health impact and on methods for sensitively assessing autophagy. The results of the paper discussed in this article highlight how the unique combination of transducer- and system design, in combination with resistive sensing enables the rapid, robust and accurate detection of the LC3 protein in autophagy. In addition to the resistive biosensor discussed here, there is currently an innovative prototype device in pre-production to measure autophagy which will be discussed in more detail in our next article.
The paper this article is based on has been published at:
Christiaan Viviers, André du Toit, Willem Perold, Ben Loos, Jan-Hendrik Hofmeyr. 2020. A Resistive Biosensor for the Detection of LC3 Protein in Autophagy. Available at https://ieeexplore.ieee.org/document/8979366.