Imagine if we could detect when a cell is under stress or determine the health of its mitochondria. This capability would be especially valuable in the context of neurodegenerative diseases like Alzheimer’s and Parkinson’s, where mitochondrial dysfunction is an early indicator. In 2015, these questions led two experts—one in cell physiology and the other in electronic engineering—to explore challenges in imaging and better analyze the three-dimensional processes within cells.
A Breakthrough in Cellular Health Assessment
After eight years of collaboration, Professors Thomas Niesler from the Department of Electrical and Electronic Engineering and Ben Loos from the Department of Physiological Sciences have developed a highly sensitive method for assessing cellular health. With their guidance, Dr. Rensu Theart created the mitochondrial event localizer (MEL) during his doctoral research on virtual reality visualization and analysis of microscopy data.
One of the key challenges in studying mitochondria is capturing their dynamic nature, as they constantly undergo fusion and fission events that occur in an instant. The MEL tool not only quantifies these events but does so within the 3D space of the cell. Theart’s work is driven by the potential impact on treating neurodegenerative diseases, where understanding these cellular processes could directly benefit patients.
Investigating the Effects of Memantine
In addition to the above, Dr Theart and Dr Sholto de Wet—a postgraduate student of Dr Loos—collaborated to investigate the effects of the Alzheimer’s drug memantine on autophagy and mitochondrial networks. Dr De Wet’s work focuses on manipulating autophagy in the context of neurodegenerative diseases, particularly Alzheimer’s disease.
Memantine, commonly prescribed for Alzheimer’s, has shown variable effectiveness, and Dr De Wet’s research found that low concentrations of the drug induce mitophagy, a process that selectively degrades dysfunctional mitochondria. This promotes the presence of healthy mitochondria, which are vital for cellular energy. At higher concentrations, memantine increases autophagy, responsible for degrading proteins.
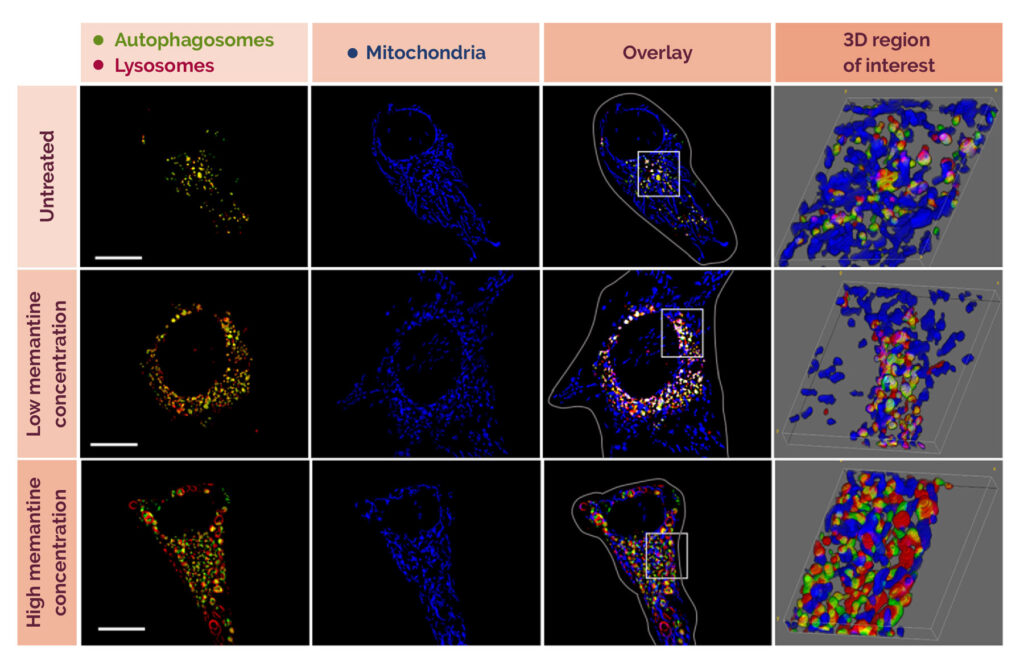
Fig. 1: The effect of two different concentrations of memantine on cells.
Further research is needed to apply these findings to more complex systems, such as the brain. Until now, research on neurodegenerative diseases has focused on misfolded protein aggregates in the brain. However, recent insights suggest a critical relationship between autophagy flux, mitochondrial network function, and lysosomal acidification—a relationship comparable to a recycling plant’s operations. Disruption in this system leads to an accumulation of harmful proteins, ultimately causing cell death.
Rethinking the Root Cause of Neurotoxicity
Drs De Wet and Loos argue that the dysfunction of the protein degradation pathway, not just the accumulation of insoluble proteins, is a key driver of neurotoxicity. By targeting any part of this interdependent system, such as enhancing mitophagy, it may be possible to protect cells from the damage caused by these proteins. Memantine could play a role in this by helping remove compromised mitochondria and associated proteins from the cell, thereby offering a new approach to treating neurodegenerative diseases.
Their research reveals a previously undescribed mechanism of memantine, showing that it can reacidify lysosomes after mitochondrial damage. This finding could lead to new treatment strategies for neurodegenerative diseases, highlighting the importance of understanding these cellular processes in developing effective therapies.
Further research is now needed to apply these findings to more complex model systems, including the brain.
Read the full article at https://www.eng.sun.ac.za/research-for-impact/